Fluorescence, Chinese word. Also known as "fluorescence", refers to a photoluminescent cold luminescence phenomenon. When a certain room temperature material by a certain wavelength of incident light (usually ultraviolet or X-ray) irradiation, absorption of light energy into the excited state, and immediately back to the excitation and emit a longer wavelength than the incident light emitted light (usually wavelengths in the visible wavelength band); a lot of fluorescent substances once the incident light stops, and the luminescence phenomenon also disappears immediately. Many fluorescent substances lose their luminescence as soon as the incident light stops. The emitted light with this property is called fluorescence. In addition, there are some substances in the incident light is withdrawn can still glow for a long time, this phenomenon is called afterglow. In daily life, people usually broadly the various kinds of weak light are called fluorescence, but not to carefully investigate and distinguish the principle of luminescence. Also refers to cold light with low temperature (not color temperature).
Principle of fluorescence generation
When light irradiates certain atoms, the energy of the light causes some electrons around the nucleus to jump from their original orbitals to orbitals with higher energy, i.e., from the ground state to the first excited single-line state or the second excited single-line state, etc. The first excited single-line state or the second excited single-line state is unstable, so it will return to the ground state. The first excited single-line state or the second excited single-line state, etc. are unstable, so they will return to the ground state. When the electrons return to the ground state from the first excited single-line state, the energy is released in the form of light, so fluorescence is produced.
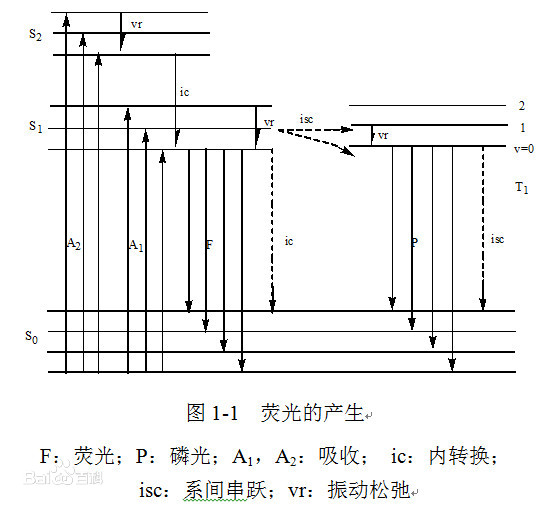
Fluorescence is the light emitted by a substance that absorbs light or other electromagnetic radiation. In most cases, the luminescence wavelength is longer and lower in energy than the absorption wavelength. However, when the absorption intensity is high, two-photon absorption may occur, resulting in a situation where the radiation wavelength is shorter than the absorption wavelength. Resonance fluorescence occurs when the wavelength of radiation is equal to the wavelength of absorption. A common example of this is the absorption of ultraviolet light by a substance that emits fluorescence in the visible wavelengths. The fluorescent lamps in our lives are based on this principle, where the phosphor coated in the lamp absorbs the ultraviolet light emitted by the mercury vapor in the lamp, and then the visible light is emitted by the phosphor to realize the visible light to the human eye.
Parameters of fluorescence
(1) Excitation spectrum: luminescent materials in different wavelengths of light excitation, the material of a spectral line and the intensity of the spectral band or luminescence efficiency and the wavelength of the excitation light.
(2) Emission spectrum: luminescent materials in a certain excitation light excitation, the intensity of its different wavelengths of light-emitting intensity changes.
(3) Fluorescence intensity: The fluorescence intensity is related to factors such as the fluorescence quantum yield, extinction coefficient and content of the substance.
(4) Fluorescence Quantum Yield Q: Quantum yield indicates the ability of a substance to convert absorbed light energy into fluorescence, and is the ratio of the number of photons emitted by a fluorescent substance to the number of photons absorbed.
(5) Stokes (Stokes) shift: Stokes shift is the difference between the maximum fluorescence emission wavelength and the maximum absorption wavelength.
(6) Fluorescence lifetime: When a beam of light excitation of fluorescent substances, the molecules of fluorescent substances absorb energy from the ground state to an excited state, and then emit fluorescence in the form of radiation to return to the ground state, the excitation stops, the fluorescence intensity of molecules reduced to the excitation of the maximum intensity of the time required for the fluorescence lifetime of 1 / e. The fluorescence lifetime of a fluorescent substance is the time required for the excitation of a fluorescent substance.

Cadmium selenide quantum dots fluoresce under ultraviolet radiation
Application areas of fluorescence
illumination
fluorescent tube
An example of this is the common fluorescent lamp. The inside of the lamp is vacuumed and filled with a small amount of mercury. The discharge of the lamp electrodes causes the mercury to emit light in the ultraviolet band. This ultraviolet light is invisible and harmful to humans. So the inside of the lamp is covered with a substance called phosphor, which absorbs that UV light and emits visible light.
Light-emitting diodes (LEDs) that emit white light are based on a similar principle. The light emitted by the semiconductor is blue, and this blue light excites the phosphorus (fluorescent) photoreceptors attached to the reflector pole, causing them to emit orange fluorescence, and the two colors of light are mixed together to approximate white light.
highlighter (pen)
Highlighter has a fluorescent agent, it encounters ultraviolet light (sunlight, fluorescent lamps, mercury lamps are more) will produce highlighter fluorescence effect, emitting white light, thus making the color look harsh fluorescent feeling. Fluorescent pen fluorescence with our watch, fluorescent stick fluorescence principle is not the same, fluorescent stick is the internal radioactive reaction, resulting in rays to stimulate the periphery of the phosphor luminescence, so they do not have any ultraviolet light at night can be luminous. The highlighter must have ultraviolet light in the case of fluorescence, which you can see very clearly as long as the highlighter's handwriting is close to the mosquito light, money detector.
Biochemical and medical
Fluorescence has a wide range of applications in biochemistry and medicine. One can stick fluorescent chemical groups onto biomolecules through chemical reactions and then sensitively detect these biomolecules by observing the fluorescence emitted by the tracer groups.
DNA sequencing graph obtained using fluorescently labeled chain terminators
Strand termination method for automated sequencing of DNA: In the original method, the primer end of the DNA needed to be fluorescently labeled in order to determine the position of the DNA ribbon on the sequencing gel plate. In the improved method, four dideoxynucleotides (ddTBP), which are chain terminators, are fluorescently labeled. After electrophoresis, DNA molecules of different lengths are separated from each other, and under ultraviolet light irradiation, fluorescence of different wavelengths is emitted from the four labeled dideoxynucleotides. By analyzing the spectrum of fluorescence, the sequence of DNA can be distinguished.DNA Detection:Ethidium bromide is a fluorescent dye, when it changes its configuration freely in solution, it can only emit weak fluorescence; when it is embedded in the nucleic acid double stranded between the base pairs and DNA molecules, it can emit strong fluorescence. Therefore, in gel electrophoresis, ethidium bromide is usually added to stain DNA.DNA microarray (biochip): fluorescent labeling of genomic probes is required, and finally the target sequence is determined by the fluorescent signal. Immunofluorescence in immunology: fluorescent labeling of antibodies, so that the site and nature of the antigen can be determined from the distribution and pattern of fluorescence. Flow cytometry (also known as fluorescence-activated cell sorter, FACS): the sample cells are fluorescently labeled and then excited by a laser beam to produce a specific fluorescence, which is then detected by an optical system and the signal is transmitted to a computer for analysis, thus obtaining a variety of corresponding characteristics of the cells. Fluorescence technology has also been applied to probe and analyze the molecular structure of DNA and proteins, especially the more complex biological macromolecules. Jellyfish luminescent protein was first isolated from the marine organism jellyfish (Aequorea victoria). It can emit green fluorescence when coexisting with Ca ions. This property has been applied to observe the flow of Ca ions in cells in real time. The discovery of jellyfish luminescent proteins spurred further study of marine jellyfish and the discovery of Green Fluorescent Protein (GFP). Green Fluorescent Protein (GFP) contains a special chromophore structure in its polypeptide chain, which can emit stable green fluorescence under ultraviolet irradiation without the need for additional cofactors or any special treatment, and has great advantages as a biomolecule or gene probe, so Green Fluorescent Protein (GFP) and its related proteins have become an important tool in the study of biochemistry and cell biology. Fluorescence Microscopy: Total Internal Reflection Fluorescence Microscopy Many biomolecules are endowed with fluorescence and can emit fluorescence without the addition of other chemical groups. Sometimes this endowed fluorescence will change with the environment, so that this environmentally sensitive fluorescence can be used to detect the distribution and nature of molecules. For example, when bilirubin binds to a special site in serum albumin, it can emit strong fluorescence. Another example is that when iron is lacking or lead is present in the red blood cells, zinc protoporphyrin is produced instead of the normal hemoglobin (hemoglobin); zinc protoporphyrin is highly fluorescent and can be used to help detect the cause of disease.
Gemstones, minerals
Gemstones, minerals, fibers, and a number of other materials that can be used as forensic evidence in a crime can emit fluorescent light of varying nature when exposed to ultraviolet light or X-rays.
Rubies, emeralds, and diamonds can fluoresce red under short wavelength ultraviolet light, as can emeralds, topazes (topazes), and pearls. Diamonds can also emit phosphorescence under X-rays.
Conceptual distinction
Luminescence caused by light (usually ultraviolet or X-rays) excitation is called photoluminescence, such as fluorescence and phosphorescence; luminescence caused by chemical reactions is called cold luminescence, concerts on the fluorescent rods is through the mixing of two chemical liquids after the occurrence of a chemical reaction luminescence; luminescence caused by the cathode rays (high-energy electron beam flow) is known as cathode ray luminescence, the fluorescence of the television tube luminescence is the cathode ray luminescence; the cold luminescence of living organisms is bioluminescence, such as the light emitted by fireflies, is "fluorescence", "fluorescence" word in ancient Chinese and "fluorescence" word generic, some of the Chinese region, the word "萤" is related to insects. In Taiwan, fluorescence is often referred to as fluorescence; in mainland China, fluorescence is often referred to as fluorescence, while "萤光" usually refers to the light emitted by fireflies.
instrumentation
To measure fluorescence you must have an instrument. The instrument that is usually used to measure the amount of fluorescence contained in a substance is called a fluorescence spectrophotometer.
Basic structure of fluorescence analyzer: excitation light source, excitation monochromator, sample chamber, emission monochromator and detection system
